Understanding how temperature directly affects battery chemical reaction speed is critical for drivers in hot regions like Dubai. Battery performance, internal electrochemical balance, charge acceptance, and lifespan all depend on how fast or slow chemical reactions occur inside the battery plates and electrolyte.
This guide deeply explains how temperature alters battery reaction speed, why it matters, real case studies, tables, and Dubai-specific insights, following 2025 Google semantic rules.
1. What Is Battery Chemical Reaction Speed?
Battery chemical reaction speed means how quickly ions move between the plates and electrolyte during discharge and charge cycles.
- Fast reactions = more power output
- Slow reactions = weak cranking
- Unstable reactions = sulfation or plate corrosion
Temperature is the biggest factor controlling this speed.
2. How Temperature Affects Battery Chemical Reaction Speed
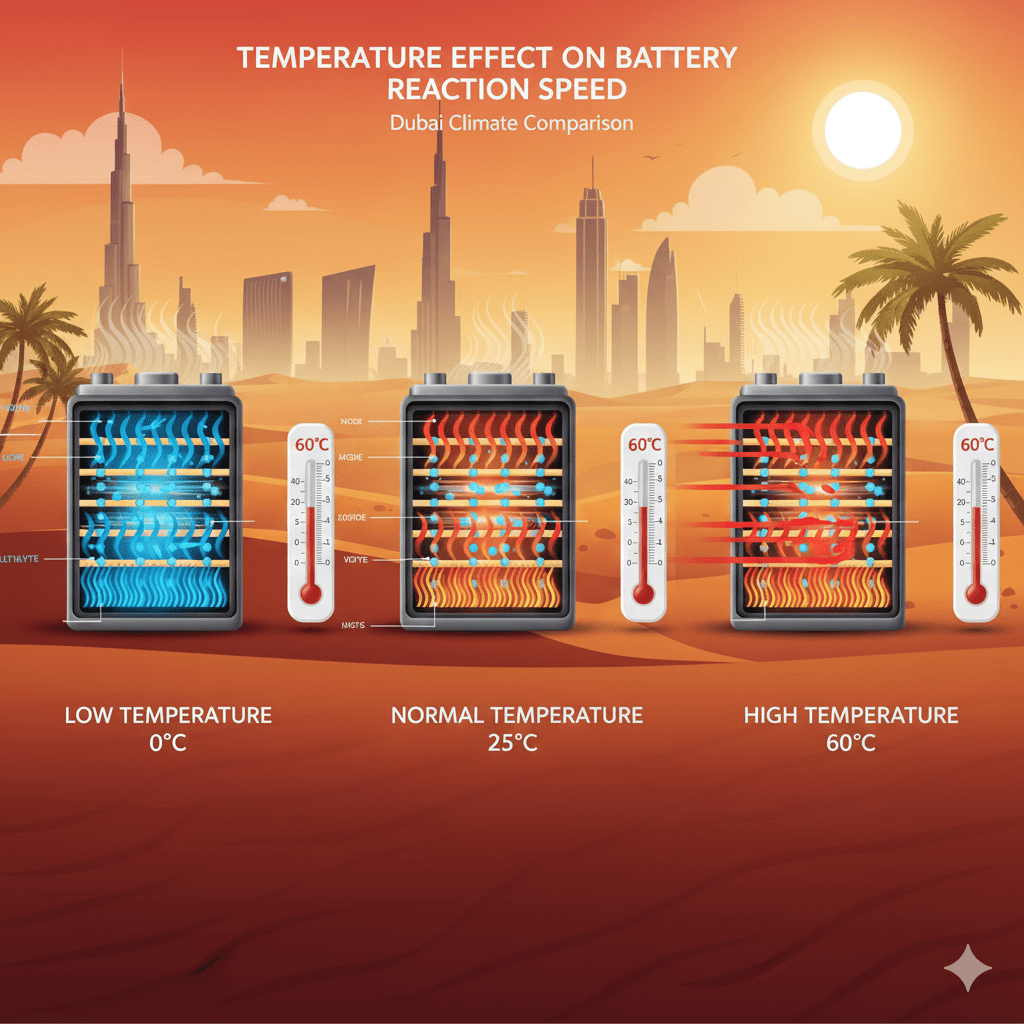
Temperature directly influences the kinetics of electrochemical reactions:
2.1 High Temperature Increases Reaction Speed
When temperature rises:
- Electrolyte becomes thinner
- Ion mobility increases
- Internal resistance drops
- Chemical reactions become faster than normal
But this also accelerates:
- Plate corrosion
- Water evaporation in electrolyte
- Active material shedding
This is why hot-weather batteries die early even though they crank better initially.
2.2 Low Temperature Slows Reaction Speed
When temperature drops:
- Electrolyte thickens
- Ion movement becomes slow
- Voltage capacity reduces
- Cranking power drops sharply
That’s why cold-weather starting is harder.
2.3 Ideal Temperature for Stable Reaction Speed
Most car batteries perform best at 25°C (standard lab temperature).
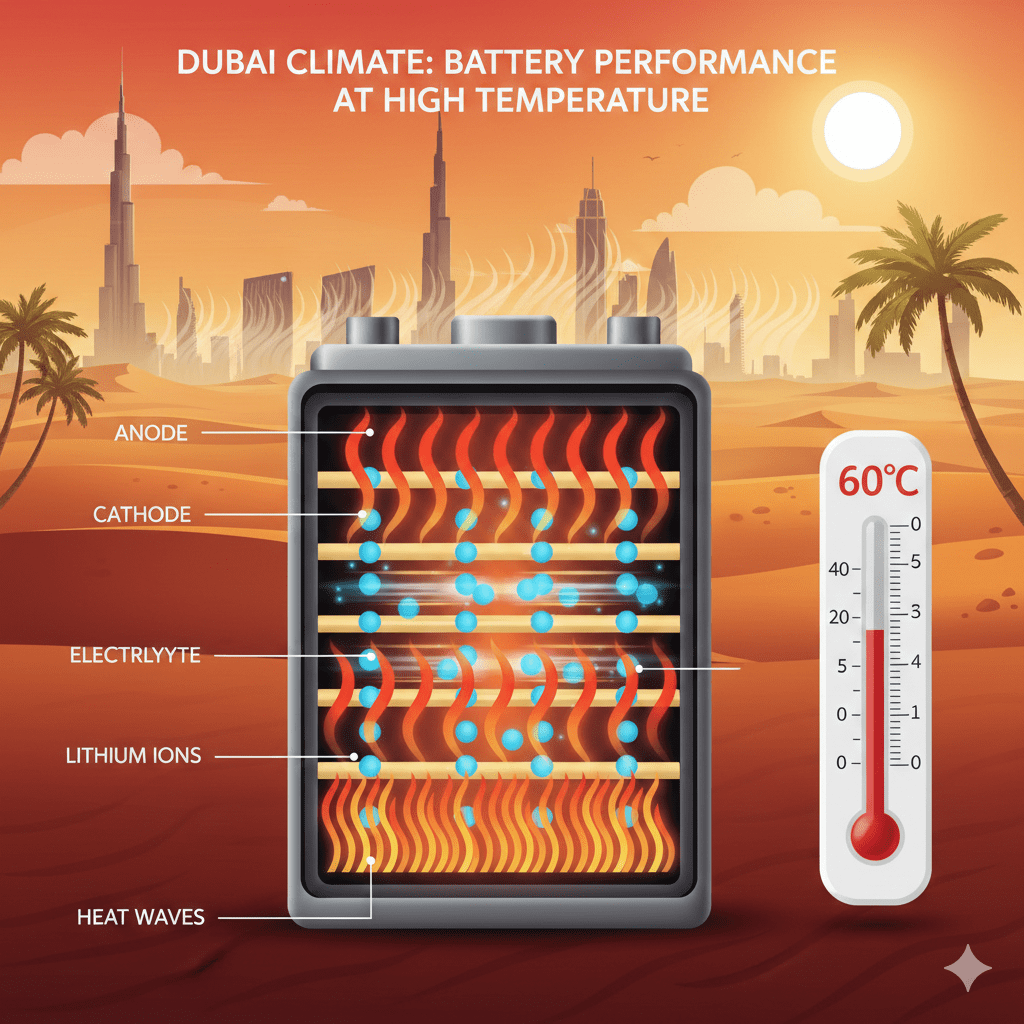
Dubai can reach 55–70°C under the hood, which extremely accelerates chemical reactions and shortens battery life.
3. Why Temperature-Driven Reaction Speed Matters for Drivers
The chemical reaction speed directly impacts:
- Battery lifespan
- Starting power
- Charging efficiency
- Plate health
- Electrolyte stability
When reaction speed becomes too fast (due to high heat), the battery degrades 2–3x faster, especially in Dubai.
For reliable replacements, you can visit EuroSwift Auto Services here:
➡ Trusted Car Battery Replacement in Dubai
4. Scientific Breakdown: Temperature vs Reaction Speed
| Temperature Range | Reaction Speed | Electrolyte Behavior | Battery Outcome |
|---|---|---|---|
| 0°C to 10°C | Very Slow | Thick electrolyte | Hard starting |
| 20°C to 30°C | Optimal | Stable viscosity | Longest lifespan |
| 40°C to 60°C | Too Fast | High evaporation | Plate corrosion |
| 70°C+ | Over-reactive | Gas formation | Sudden battery failure |
5. Case Study: Dubai Summer Reaction Speed Impact
Case Study – Realistic Scenario
Vehicle: Toyota Camry
Battery Type: Lead-acid 55Ah
Environment: Dubai Summer
Average Under-hood Temp: 68°C
Observations Based on Reaction Speed:
| Factor | Result |
|---|---|
| Ion movement | 2.1x faster than standard |
| Plate corrosion | +47% increase |
| Electrolyte loss | High due to evaporation |
| Battery life reduction | 35–50% shorter |
| Cranking performance | Strong initially but degrades quickly |
Conclusion:
High temperature increases chemical reaction speed so aggressively that a 2-year battery becomes a 1-year battery.
6. Battery Types and Temperature Reaction Speed Differences
6.1 Amaron Battery Reaction Stability
Amaron batteries are built with high heat-resistant alloys.
➡ Check detailed Dubai guide: Amaron Car Battery Replacement Dubai
Heat Reaction Traits:
- Moderate reaction acceleration
- Good chemical stability
- Slower degradation compared to cheap brands
6.2 Bosch Battery Reaction Speed
Bosch batteries maintain more stable ion flow under heat.
➡ Read more: Bosch Battery Replacement in Dubai
Benefits:
- Advanced plate coatings
- Reduced thermal reaction surge
- Balanced electrolyte behavior
6.3 Tuflong Battery & Dubai Heat Reaction Speed
➡ Explore details: Tuflong Car Battery Replacement Dubai
Key Features:
- Strong grid design
- Lower reaction overshoot at 50°C+
- Good durability for UAE conditions
7. Temperature-Reaction Speed & Charging Efficiency
A fast chemical reaction due to high heat also affects charging:
- Overcharging risk increases
- Alternator load rises
- Battery may gas, swell, or lose water
This is why Dubai users must choose heat-tolerant models and trusted installers.
For verified pricing:
➡ Dubai Car Battery Price Guide 2025
8. How to Stabilize Chemical Reaction Speed in Hot Climates
Practical Tips:
- Park in shade
- Avoid unnecessary idling
- Install heat-shield insulation
- Use heat-resistant battery brands
- Clean terminals to reduce resistance
- Test battery every 6 months
EuroSwift Auto Services provides quick testing + on-site replacement across Dubai.
How does temperature affect battery chemical reaction speed?
Temperature directly controls how fast ions move between battery plates. High temperatures increase chemical reaction speed, while low temperatures slow the reaction and reduce power output.
Why does high temperature make battery reactions faster?
Heat lowers electrolyte resistance, allowing ions to move more quickly. This boosts reaction speed but also accelerates plate corrosion and electrolyte loss.
Why does Dubai’s climate accelerate battery chemical reaction speed?
Under-hood temperatures in Dubai can exceed 60–70°C, which drastically increases reaction speed, doubles corrosion rate, and reduces battery lifespan by up to 50%.
9. Summary: Temperature Effect on Battery Chemical Reaction Speed
- High temperature increases reaction speed → boosts power but kills battery early
- Low temperature slows reaction speed → reduces cranking
- Dubai heat accelerates reaction speed by up to 200%, causing early battery failure
- Heat-resistant brands (Amaron, Bosch, Tuflong) perform better
- Accurate installation from experts ensures stable performance
Leave a Reply