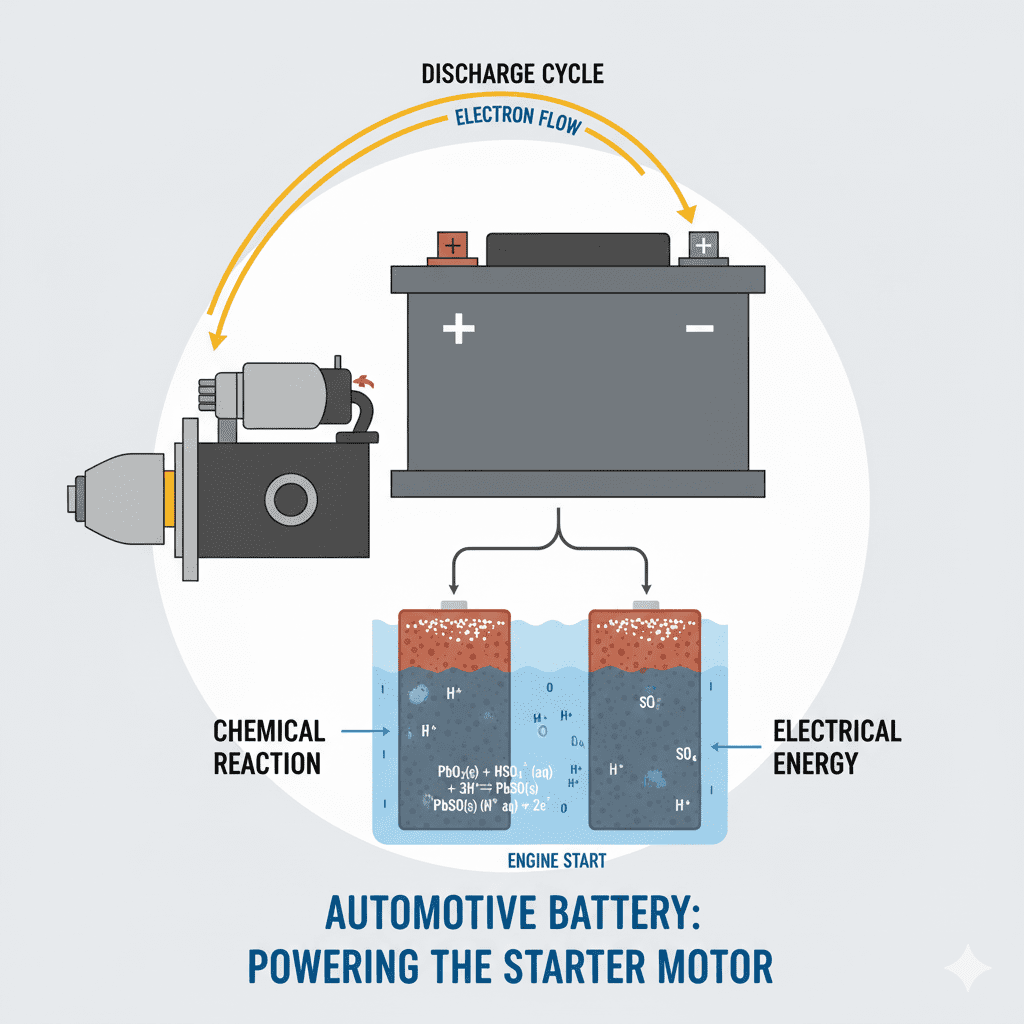
A car engine starts because the car battery generates electrical power through a controlled chemical reaction. This reaction converts stored chemical energy into electrical energy, and that energy is used to rotate the engine, ignite fuel, and power all critical components during the start cycle.
This guide explains exactly how a car battery generates power for the engine, including electrochemical reactions, electron flow, cranking power, starter motor load, voltage behavior, internal resistance, power delivery sequence, engine ignition energy, alternator recharge, case study, tables, and internal anchors.
How Power Generation Starts Inside the Battery
Car batteries generate power using an electrochemical process. Inside the battery:
- Lead plates and lead dioxide plates react
- Sulfuric acid electrolyte releases ions
- Electrons move through the circuit
- Voltage is produced due to chemical potential
- High current becomes available instantly
This chemical-to-electrical conversion produces DC power, which is required by the engine’s starter system.
The Chemical Reaction That Creates Power
During engine start:
Pb + PbO₂ + 2H₂SO₄ → 2PbSO₄ + 2H₂O + Electrical Energy
This reaction produces:
- Free electrons
- Voltage pressure
- High current output (cranking amps)
Those electrons and current flow out of the positive terminal toward the starting system.
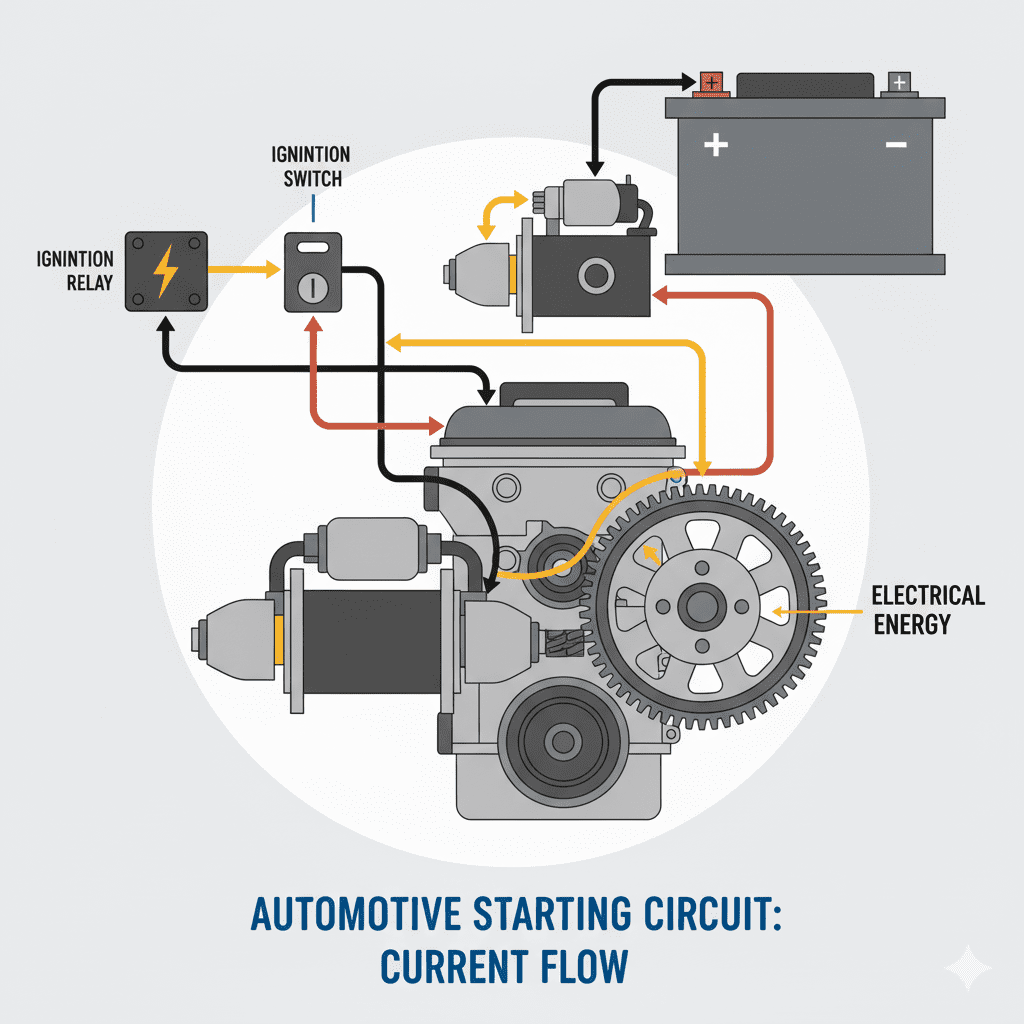
How the Battery Sends Power to the Engine
When you turn the key or press Start:
- Battery releases stored electrical energy
- Current flows to the starter motor
- Starter motor rotates the flywheel
- Flywheel rotates the crankshaft
- Engine combustion starts
- Alternator begins recharging the battery
This entire sequence happens within 1–2 seconds, powered entirely by the battery.
Why High Current Is Needed to Start the Engine
The engine does not start with just voltage — it needs massive amperage to turn a heavy mechanical system.
Battery power is required to:
- Spin the starter motor
- Compress air–fuel mixture
- Activate ignition coils
- Trigger fuel pump
- Power sensors & ECU
A weak battery cannot supply this “starting surge,” resulting in slow cranking.
Electron Flow: The Real Source of Engine-Starting Power
Battery power reaches the engine through this path:
Battery → Starter Relay → Starter Motor → Engine Flywheel → Ground → Battery Negative Terminal
This continuous electron loop generates the torque needed to rotate the engine.
Voltage & Current Behavior During Engine Start
| State | Voltage | Action |
|---|---|---|
| Rest | 12.6V | Fully charged |
| During Start | Drops to 9.6V+ | Normal crank |
| Below 9.6V | Weak output | Battery underperforming |
If voltage collapses under load → the battery cannot generate enough power.
Internal Resistance & Power Generation
Low internal resistance = strong engine-starting power
High internal resistance = weak crank, low voltage
Internal resistance increases due to:
- Sulfation
- Plate corrosion
- Low charge cycles
- Heat exposure (common in Dubai)
Table: How Engine Components Use Battery Power
| Engine Component | Battery Power Role |
|---|---|
| Starter Motor | Converts electrical → mechanical power |
| Ignition Coil | Produces spark voltage |
| ECU/ECM | Runs engine control logic |
| Fuel Pump | Supplies pressurized fuel |
| Sensors | Communicate engine data |
All depend on a stable battery power supply.
Case Study: Engine Failing to Start Due to Weak Battery Power (Dubai)
Car: Honda Civic 2016
Symptom: Crank + click sound
Cause: Battery couldn’t generate required power
Weather: 45°C (high heat zone)
Diagnostics by EuroSwift Auto Services
| Test | Result | Interpretation |
|---|---|---|
| Battery Voltage Rest | 12.0V | Low charge |
| Cranking Voltage | 8.7V | Weak power |
| CCA Test | 42% health | Poor current delivery |
| Electrolyte | Low density | Weak reaction |
Conclusion:
Battery failed to generate enough power to rotate the engine due to heat-related sulfation and low chemical activity. Replaced with heat-resistant Amaron battery.
EuroSwift Auto Services
To understand replacement solutions:
👉 Car battery replacement near me in Dubai
For stronger power-generating batteries:
👉 Amaron battery replacement Dubai
👉 Bosch battery replacement Dubai
👉 Tuflong battery replacement Dubai
To compare power-efficiency prices:
👉 Car battery price guide Dubai 2025
How does a car battery actually generate power for the engine?
A car battery generates power through a chemical reaction between lead plates and sulfuric acid, creating electrical energy. This electricity powers the starter motor, helping the engine crank and start.
What chemical process helps car batteries supply energy to the engine?
The main process is electrochemical conversion. Lead dioxide (positive plate) and sponge lead (negative plate) react with sulfuric acid, releasing electrons, which flow through the cables to start the engine.
How can I improve battery power output for better engine starting?
Keep terminals clean
Ensure proper electrolyte levels (for non-maintenance-free batteries)
Install a high-performance battery like Amaron, Bosch, or Tuflong
Get routine battery checks every 6 months
You can explore premium options here:
Amaron Battery Replacement
Bosch Battery Replacement
Tuflong Battery Replacement
Conclusion
Car batteries generate power for the engine by converting chemical energy into electrical energy, delivering high current to the starter motor, powering ignition components, and enabling the engine to rotate. Without this instant power surge, the engine cannot start.
For expert testing, diagnosis, or replacement, EuroSwift Auto Services provides reliable service in Dubai.
Leave a Reply