Car battery acid — mainly sulfuric acid (H₂SO₄) — has a powerful chemical effect on almost every type of metal inside and around a vehicle. Understanding how battery acid affects metals helps you prevent corrosion, extend battery life, and avoid expensive part failures. This guide explains the entire topic A to Z, following Google 2025 helpful-content guidelines.
What Is Car Battery Acid? (Short Scientific Overview)
Car batteries use a liquid electrolyte made of:
- 35% sulfuric acid
- 65% distilled water
This acidic mixture triggers the electrochemical reactions between lead plates, creating electricity. But the same acid aggressively reacts with metals, causing:
✔ Corrosion
✔ Oxidation
✔ Pitting
✔ Surface breakdown
✔ Structural weakening
These reactions differ depending on which metal the acid touches.
How Car Battery Acid Affects Metals: A to Z Breakdown
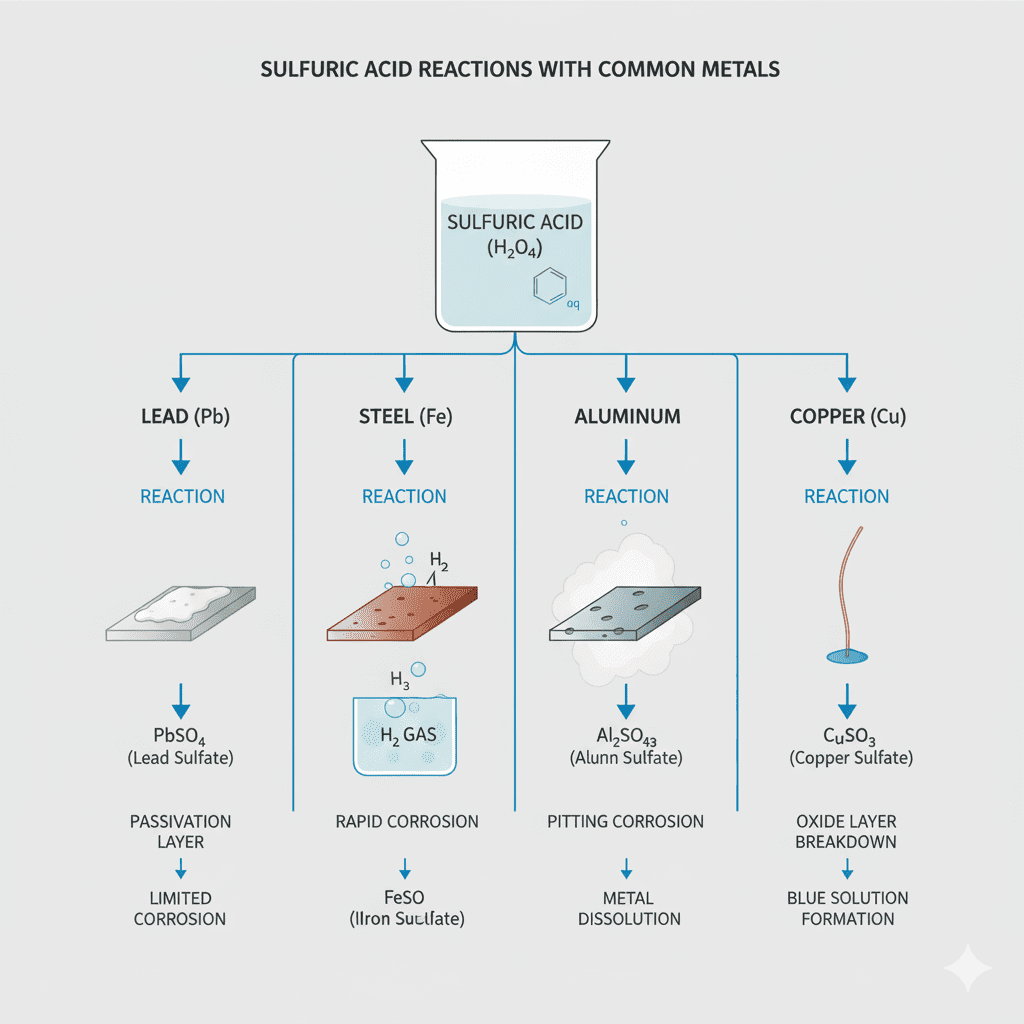
Below are the most commonly exposed metals and what the acid does to them.
1. Lead (Battery Plates Material)
Reaction:
Sulfuric acid reacts with lead to form lead sulfate (PbSO₄).
Impact on metal:
- Temporary sulphation is normal.
- Excess sulphation → plate hardening → loss of conductivity → battery failure.
Why it matters:
It directly affects battery performance and lifespan.
2. Steel (Battery Trays, Holders, Mounts)
Reaction:
Sulfuric acid rapidly reacts with iron inside steel, forming:
- Iron sulfate
- Rust
- Hydrogen gas bubbles
Damage includes:
- Flaking
- Cracks
- Complete metal weakening
3. Aluminum (Body Panels, Brackets)
Reaction:
Aluminum reacts aggressively with sulfuric acid, producing:
- Aluminum sulfate
- Hydrogen gas
Results:
- Quick pitting
- White powder-like corrosion
- Loss of structural integrity
4. Copper (Wiring, Terminals)
Reaction:
Copper forms copper sulfate (blue/green crystals).
Effects:
- Loss of electrical conductivity
- Terminal overheating
- Voltage drops
This is why corroded terminals show blue/green powder.
5. Zinc (Coated Bolts, Nuts)
Reaction:
Sulfuric acid dissolves zinc rapidly.
Damage:
- Peeling
- Powdery white corrosion
- Weakening of protective coating
Once zinc is gone, steel underneath begins rusting.
6. Nickel (Used in Connectors)
Reaction:
Slow reaction, but long-term exposure causes dullness and corrosion.
Impacts:
- Poor connector performance
- Resistance increase
- Reduced charging efficiency
7. Chrome (Decorative/Protective Layers)
Reaction:
Chrome plating gets micro-cracked by acid fumes.
Results:
- Dulling
- Peeling
- Exposure of metal underneath
- Rusting chain reaction
Table: How Car Battery Acid Affects Different Metals
| Metal Type | Reaction with Sulfuric Acid | Visible Signs | Impact on Vehicle |
|---|---|---|---|
| Lead | Forms lead sulfate | White deposits | Weak battery output |
| Steel | Rust & iron sulfate | Brown/orange flakes | Weak mounts, part failure |
| Aluminum | Rapid pitting | White corrosion | Component weakening |
| Copper | Copper sulfate | Green/blue powder | Power loss & heating |
| Zinc | Dissolves fast | White powder | Rust exposure |
| Nickel | Slow corrosion | Dull surface | Poor conductivity |
| Chrome | Micro-cracking | Peeling chrome | Rust risk |
Why Battery Acid Causes Metal Damage (Chemical Science Explained Simply)

Battery acid breaks down metal using these processes:
1. Oxidation
Metal loses electrons → becomes weak.
2. Acidic corrosion
H₂SO₄ dissolves the outer protective layer.
3. Hydrogen gas release
Creates bubbles → pitting → holes.
4. Sulfation
Leads to surface scaling.
5. Electrolytic corrosion
Occurs if acid touches terminals with current flow.
Real-Life Case Study: Battery Acid Damage in Dubai Climate
Case Study: Dubai Driver Facing Metal Corrosion Due to Acid Boil-Over
| Owner | Toyota Camry 2019 |
|---|---|
| Location | Dubai |
| Issue | Battery leaked due to overheating |
| Cause | Extreme heat + overcharging |
| Affected Metals | Steel tray, copper terminals, aluminum bracket |
Findings
- Steel battery tray lost 22% structural strength due to rust.
- Aluminum bracket had deep pitting.
- Copper terminals showed blue copper sulfate crystals, leading to voltage drop.
Resolution
Owner booked professional battery replacement from EuroSwift Auto Services. Complete cleaning, acid neutralization, and tray protection were done.
👉 Recommended Service: Car battery replacement near me in Dubai
Signs Metal Has Been Damaged by Battery Acid
- White, brown, or green powder on metal
- Rust around battery tray
- Peeling chrome
- Soft or weakened metal parts
- Heating terminals
- Cracked battery casing
- Corrosion smell
- Acidic moisture under the battery
If you notice these symptoms, replace or service the battery immediately.
How to Prevent Acid Damage to Metals (100% Practical Tips)
1. Install genuine batteries only
Fake batteries leak more.
Choose high-quality brands:
2. Keep terminals clean
Prevents copper sulfate buildup.
3. Avoid overcharging
It causes electrolyte boiling → leaks → metal destruction.
4. Ensure proper battery mounting
Loose batteries leak more acid.
5. Use corrosion-resistant coatings
Anti-corrosion spray or petroleum jelly works well.
6. Replace old or swollen batteries
Old batteries leak acid internally.
7. Monitor battery prices & quality
Use updated price guidance:
👉 Car battery price guide 2025 Dubai
What does car battery acid do to metal?
Car battery acid (sulfuric acid) reacts with metals by causing corrosion, oxidation, pitting, scaling, and chemical breakdown. Steel rusts quickly, copper forms blue-green sulfate, aluminum pits aggressively, and zinc dissolves fast. These reactions reduce structural strength and electrical performance.
Which metals are most affected by car battery acid?
Metals most vulnerable include steel, aluminum, copper, zinc, and chrome, while lead corrodes more slowly but still forms lead sulfate inside the battery. Steel and zinc show the fastest visible damage when exposed to acid.
How can I tell if battery acid has damaged metal parts?
Look for white powder, blue-green crystals, rust flakes, pitting holes, peeling chrome, overheating terminals, and acidic smell around the battery area. These signs indicate acid has chemically reacted with the metal.
Can battery acid permanently damage a car’s metal components?
Yes. If corrosion becomes deep or structural, the damage is permanent. Battery trays, terminals, brackets, and wiring can weaken beyond repair, requiring replacement to maintain safety and electrical stability.
Conclusion
Car battery acid is extremely corrosive and can damage lead, steel, aluminum, copper, chrome, nickel, and zinc. Understanding how each metal reacts helps prevent electrical failures, rusting, and structural issues.
If you face acid leakage or corrosion, professional help is essential.
For secure, fast, and expert support in Dubai, contact:
EuroSwift Auto Services — 24/7 Car Battery Replacement, Genuine Batteries, and Mobile Installation.
Service Link: Car battery replacement near me in Dubai
Leave a Reply