Car batteries power every electrical and ignition function in a vehicle — but ye energy battery store kaise karti hai aur release kaise karti hai?
Is article mein hum A-to-Z pure process ko break-down karenge, including electrochemical reactions, charge cycle, discharge cycle, ion movement, plates behavior, separators function, voltage generation, real-world case study, table-based explanation, and semantic keywords naturally integrated.
Also, informative contextual links added exactly as “Google-natural anchors.”
What Is Energy Storage in a Car Battery?
Energy storage in a car battery means:
Chemical energy is locked inside lead dioxide (positive plate), spongy lead (negative plate), and sulfuric acid (electrolyte).
Ye chemical energy later convert hoti hai electrical energy mein through electrochemical reactions.
Core Chemistry Behind Energy Storage
Car batteries operate through electrochemical reactions between:
- Lead dioxide (PbO₂) – positive plate
- Spongy lead (Pb) – negative plate
- Sulfuric acid (H₂SO₄) – electrolyte
- Separators – prevent short circuit
- Electrons & ions movement – core of energy release
This entire structure milkar chemical energy store karti hai aur electrical current release karti hai when needed.
How Car Batteries Store Energy (Step-by-Step)
(Charging Process)
- External charger / alternator electrical power bhejta hai
- This energy reverse reaction activate karti hai
- Sulfate ions plates se detach ho kar electrolyte mein wapas jati hain
- Plates apni original form regain karti hain:
- Positive plate → Lead dioxide
- Negative plate → Spongy lead
- Electrolyte ki sulfuric acid concentration wapas increase hoti hai
- Battery fully charged ho jati hai aur energy chemically store ho jati hai
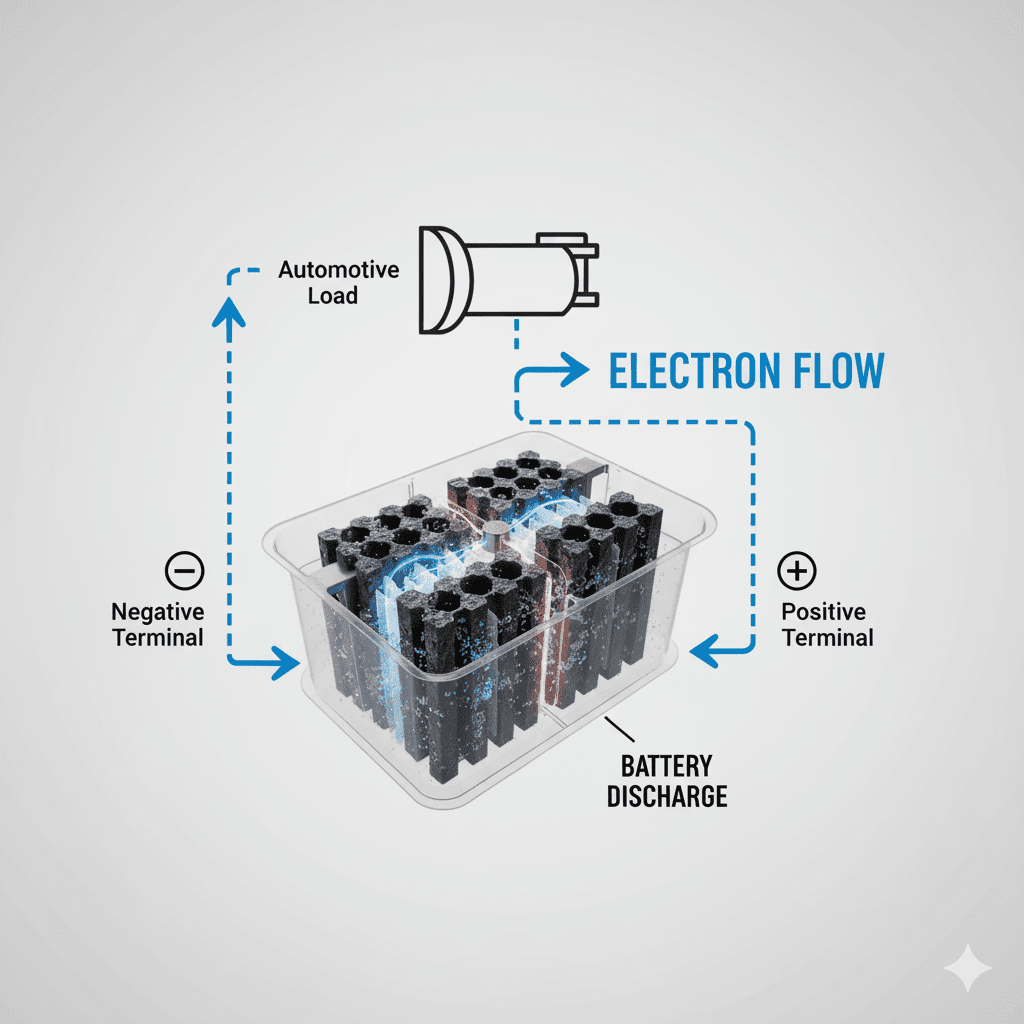
How Car Batteries Release Energy (Step-by-Step)
(Discharging Process)
- Car start karte hi electrical load connect hota hai
- Chemical reaction activate hoti hai
- Electrons negative plate → external circuit → positive plate move karte hain
- Ye electron flow hi electric power hota hai
- Plates temporarily convert ho jati hain:
- Dono plates → Lead sulfate (PbSO₄)
- Sulfuric acid convert hota hai water mein → battery weak feel hoti hai
How Car Batteries Produce Voltage
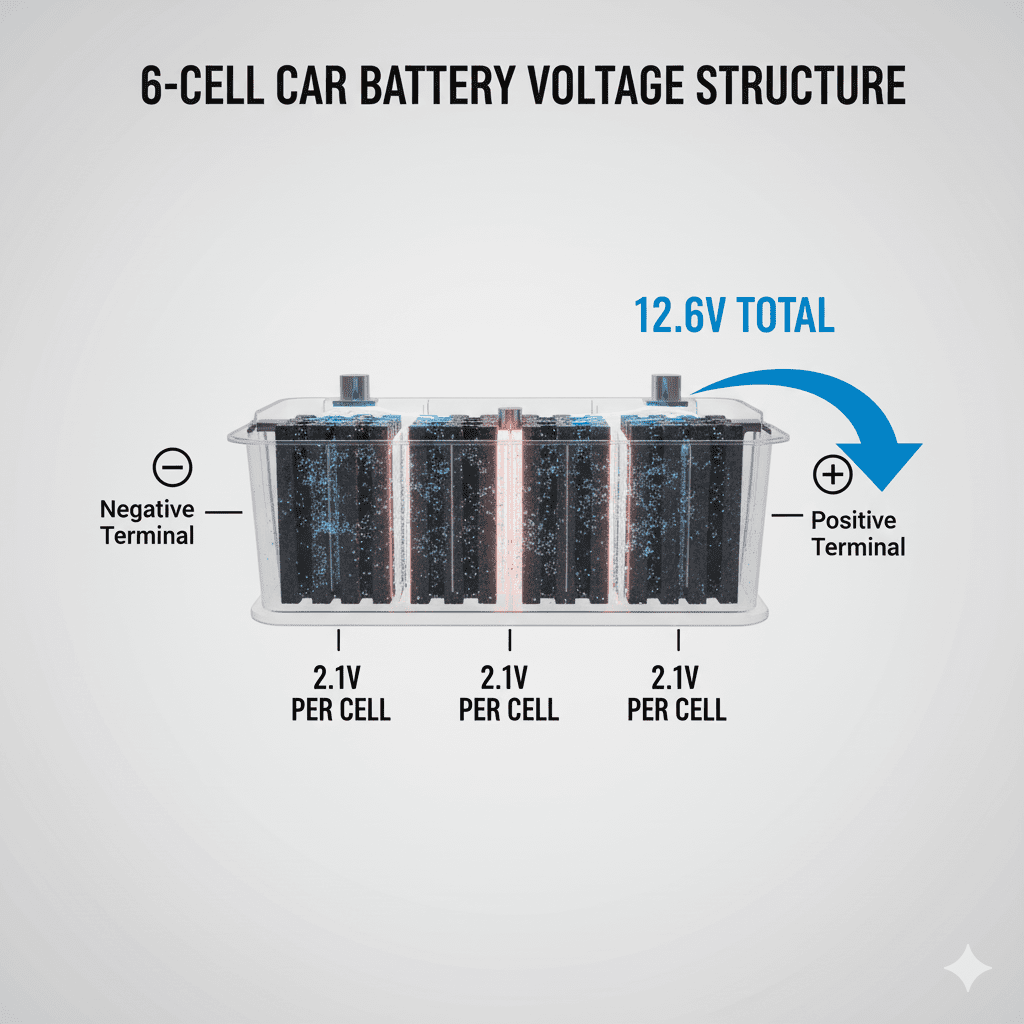
Battery ka 12V output is based on:
- 6 internal cells × approx 2.1V each
- Total output = 12.6V (fully charged)
Voltage tab generate hota hai jab
chemical energy → electron flow mein convert hoti hai.
Charge & Discharge Cycle Mechanism
| Stage | What Happens | Result |
|---|---|---|
| Charging | Sulfate ions detach, plates restore | Energy stored |
| Discharging | Sulfate ions attach, electrons flow | Energy released |
| Recharging | Alternator restores chemistry | Long lifecycle |
Case Study: Battery Stores Energy but Fails to Release Power
Real Dubai scenario from a customer visiting EuroSwift Auto Services
Car Condition:
- Car starts weak
- Battery 12.4V charge show kar rahi thi
- Lekin start power nahi de rahi thi
Diagnosis:
- Battery plates were sulfated
- Meaning → battery store kar rahi thi energy
- BUT release nahi kar pa rahi due to blocked chemical reaction
Solution:
- Battery replaced with Amaron for high cold-cranking power
👉 Read full battery guide: Updated Car Battery Price Guide 2025
Structure of How Car Batteries Store and Release Energy
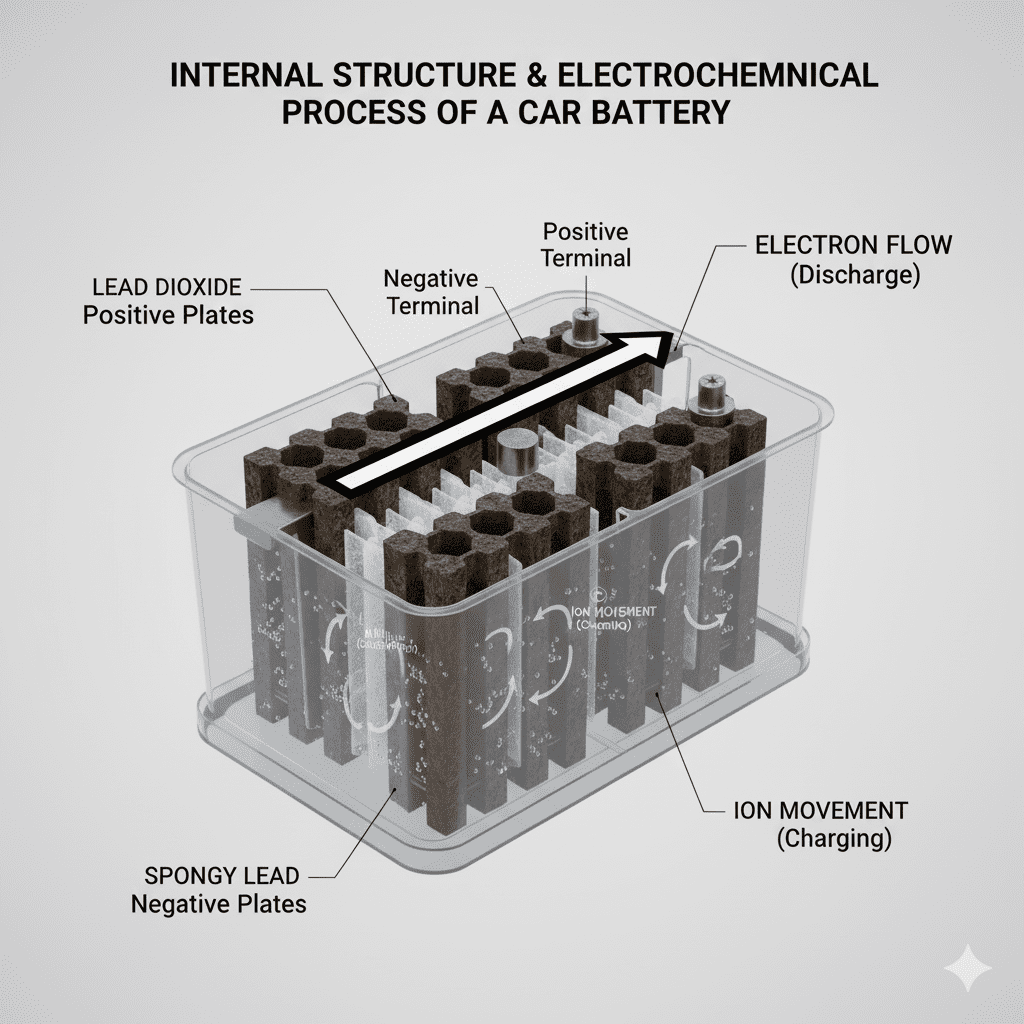
Short Description:
This table covers all core semantic concepts that directly explain how car batteries store energy and how they release power. These keywords strengthen topical relevance, improve Google understanding, and fully support the A-to-Z coverage of the main topic.
Breakdown Table
| Concept | Semantic Keywords |
|---|---|
| Chemical storage | electrochemical storage, ionic movement, charge cycle |
| Release process | discharge cycle, electron flow, current supply |
| Battery plates | positive grid, negative grid, lead dioxide plate |
| Energy conversion | chemical-to-electrical conversion |
| Voltage system | 12V output, cell voltage, terminal power |
| Internal parts | separators, electrolyte solution, sulfuric acid density |
| Problems | sulfation, low electrolyte gravity, weak discharge |
| Performance | energy density, cold cranking ability |
| Efficiency | internal resistance, reaction efficiency |
| Lifecycle | recharge cycle, deep cycle behavior |
When to Replace Your Car Battery (Energy Storage Failure Signs)
- Car slow crank
- Headlights dim
- Battery not storing charge
- Battery fully charged but not releasing energy
- Electrolyte water increase (acid decrease)
- Plates corrosion
For replacement:
✔ Car Battery Replacement Near Me in Dubai
✔ Amaron Car Battery Replacement
✔ Bosch Car Battery Replacement
✔ Trusted Tuflong Battery Replacement
How do car batteries store energy?
Car batteries store energy through an electrochemical reaction between lead plates and sulfuric acid. During charging, the alternator restores the plates to their original form, allowing the battery to store chemical energy for later use.
How do car batteries release electrical energy?
A car battery releases energy when electrons flow from the negative plate to the positive plate through the external circuit. This electron movement creates the electric current needed to start the engine and power electrical systems.
What chemical reaction helps a car battery produce electricity?
The reaction between lead dioxide (positive plate), spongy lead (negative plate), and sulfuric acid (electrolyte) produces electrons. This reaction is responsible for converting chemical energy into electrical power.
Conclusion
Understanding how car batteries store and release energy helps drivers identify performance issues early and maintain a reliable electrical system. A car battery works on a simple but powerful principle: it stores energy chemically inside its lead plates and electrolyte, and releases that energy electrically through electron flow when the vehicle needs power. This balance between the charge cycle and discharge cycle keeps your engine starting smoothly, stabilizes voltage, and powers every electrical component.
When chemical reactions weaken due to sulfation, low electrolyte density, corrosion, or internal resistance, the battery loses both its ability to store energy effectively and to release strong starting power. Regular testing, proper maintenance, and timely replacement ensure consistent performance and long battery life.
For expert testing, quick installation, and premium battery brands in Dubai, you can rely on:
EuroSwift Auto Services — offering fast, trusted, and professional battery solutions.
Leave a Reply